Example of Solution That Can Find at Home
Common Examples of Solutions: Science in Everyday Life
It's easy to find examples of solutions in everyday life. Chances are that there are many types of solutions in your home, your school and other locations you visit regularly.
 solutions example
solutions example
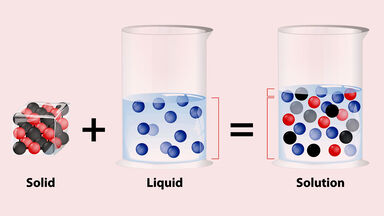
What Is a Solution in Science?
Most solutions are made when more than one gas, solid or liquid is dissolved in a liquid. Some solutions are combinations of two or more gases, or two or more liquids or even two or more solids. All solutions are homogeneous.
- Homogeneous means that the two (or more) substances combine in such a way that the mixture is the same all throughout.
- Nothing settles to the bottom of the container a solution is in. The individual substances that formed it cannot be physically separated, even by using a filter.
Examples of Solutions in Everyday Life
There are several types of solutions. You have probably seen or studied examples of each type, as they are very common.
Liquid/Liquid Solutions
Many household liquids and automotive products are examples of liquid/liquid solutions.
- antifreeze - The substance that keeps a car's radiator from freezing up during the winter is a solution of water and ethylene glycol.
- mouthwash - The minty liquid that keeps your breath smelling fresh is one or more chemicals, such as cetylpyridinium chloride, dissolved in water.
- household cleaning products - Cleaning liquids like Windex, Formula 409 and others are solutions of water and various chemical substances.
- vinegar - The vinegar used to pickle vegetables and in various cleaning solutions is a combination of water and acetic acid.
- disinfectants - Many disinfectants, including Lysol and hand sanitizer, are a mixture of ethanol and water.
- hydrogen peroxide - The hydrogen peroxide used for household purposes is an extremely diluted solution of pure hydrogen peroxide in water.
- liquid soap - Hand soap, liquid dishwashing soap and liquid laundry detergent are solutions of various compounds in water.
Solid/Liquid Solutions
There are many examples of solid/liquid solutions in everyday life.
- pancake syrup - The syrup that you like to eat on pancakes or waffles is a solution of sugar in water along with flavoring agents.
- sports drinks - Sports drinks like Gatorade and Powerade are solutions of salt, sugar and other ingredients dissolved in water.
- sweetened tea or coffee - When sugar is dissolved into brewed tea or coffee, the beverage becomes a solution.
- salt water - If you've ever had to gargle with warm salt water to help with a sore throat, you have created a solution by dissolving salt (sodium chloride) in water.
- ocean water - The water in the oceans and seas, as well as other bodies of water that are not fresh water, is saltwater that occurs naturally.
Gas/Liquid Solutions
There aren't as many types of gas/liquid solutions as there are liquid/liquid or liquid/solid ones, but you're probably very familiar with them.
- carbonated beverages - Sparkling water and club soda are solutions of water and carbon dioxide.
- soft drinks - Soft drinks like Coca Cola and Pepsi are also solutions of water and carbon dioxide along with sugar and other flavoring agents.
- ponds - Oxygen gas is dissolved in ponds, as well as other bodies of water that support life.
- aquariums - If you have a fish aquarium, you have a small body of water that supports life in your home. Aquarium pumps oxygenate the water in a fish tank.
Gas/Gas Solutions
While there aren't many common examples of gas/gas solutions, there are a few really important ones.
- air - The air that you breathe is a solution of oxygen dissolved in nitrogen, both of which are gases.
- natural gas - Natural gas, such as the gas used by the stove or heater in a home, is a solution of ethane, butane and propane dissolved in methane gas.
Solid/Solid Solutions
Metal alloys are examples of solid/solid solutions. An alloy is a metal that is made by combining two or more metals together.
- bronze - This common metal is actually an alloy of tin and copper. Many homes have bronze door knockers, light fixtures or other design elements.
- sterling silver - This common metal is actually an alloy of silver and copper. It is commonly used to make jewelry and upscale serving pieces or utensils.
- pewter - Modern pewter is a combination of multiple metals. It is mostly tin and typically contains antimony, copper and bismuth.
Solvent vs. Solute
Every solution is a combination of at least one solvent and solute.
- solvent - The substance that makes up the majority of the solution is the solvent. It is what the other substance(s) dissolves in. Water is the most common solvent.
- solute - The solute (or solutes) in a solution are the substances (like salt or sugar) that dissolve in the solvent. There is less solute than solvent.
Consider vinegar as an example. For vinegar, the solvent is water and the solute is acetic acid. Look at the label on a bottle of vinegar in your cupboard or the next time you go to the grocery store. Notice the acidity percentage on the label. A vinegar solution with five percent acidity is five percent acetic acid and 95 percent water (by weight).
Solutions vs. Suspensions and Colloids
A mixture is formed when two or more substances are combined. Solutions, suspensions and colloids are all examples of mixtures.
Solutions
As discussed above, solutions are homogeneous mixtures that will not separate or settle over time. Once the substances are combined, they stay combined.
Suspensions
Suspensions differ from solutions in that they are heterogeneous mixtures. Heterogeneous mixtures are not the same all throughout and they will settle out over time. A suspension is basically a lot of small particles suspended by the water, rather than being dissolved in it, so it must be shaken frequently. Spray paint is an example of a suspension.
Colloids
Colloids are a special case of mixture, somewhat between solutions, which don't settle, and suspensions, which settle out quickly. Colloid mixtures are not solutions, but they do take a long time to settle out. A gallon of oil paint is a good example of a colloid. It will eventually settle, but will take months to do it.
Learn More About Mixtures in Science
In science, a mixture is just a combination of any two (or more) substances that do not chemically bond with each other when combined. Now that you know that solutions, suspensions and colloids are examples of mixtures, take the time to learn more about this important scientific topic.
Explore different types of solutions, such as saturated solutions. Then, move on to discovering hypertonic solutions. Finally, review these examples of mixtures to deepen your knowledge and discover other types of mixtures.
Example of Solution That Can Find at Home
Source: https://examples.yourdictionary.com/common-examples-of-solutions-science-in-everyday-life.html